What is a quantum particle really like? It’s not what you think

Quantum mechanics is known for some very mind-bending claims, like cats being simultaneously dead and alive, and electrons and protons and other denizens of the subatomic world being both particles and waves. It’s quite confusing. But, using modern ideas of the quantum world, there are ways to envision exactly what is going on. In brief, particle interactions are a heady mix of vibrating and interacting fields.
Classical particles and waves
The concept of a classical particle is familiar. A particle is an object with an identifiable location. The object could be big or small, or it could have a peculiar shape. For a subatomic particle like an electron, the usual mental image is something akin to a microscopic ball. When particles interact, they can bounce off one another, like two billiard balls, or can merge, like two lumps of clay hitting one another.
Classical waves are equally familiar. Think of the up and down wiggles on the surface of a lake as a series of objects are dropped into it. Mathematically, a one-dimensional wave is just a steadily oscillating sinusoidal curve. It extends infinitely in either direction with a fixed, repeating wavelength. Unlike particles, waves have no identifiable location. Furthermore, waves interact very differently than particles. As two waves interact, they pass through one another, with the crests and troughs of the two waves either enhancing each other into a bigger crest or cancelling each other entirely (known as constructive and destructive interference, respectively).
Given that traditional particles and waves seem to have such very different properties, it is easy to understand how early 20th century physicists were so confused as they tried to reconcile claims that things like photons and electrons were both particles and waves. However, scientists have come to understand that subatomic objects have both wave and particle properties, rather than existing as one or the other.
Wave-particle duality
For example, an object like an electron has a wavelength, but it doesn’t extend off to infinity. Instead, the amplitude (or height) of the wave has a location where it is maximized, and then it decreases at distances farther from the maximum. The result is what is called a wave packet. In the context of early 20th century quantum mechanics, the term “wavicle” was briefly in vogue, although it is now rarely used.
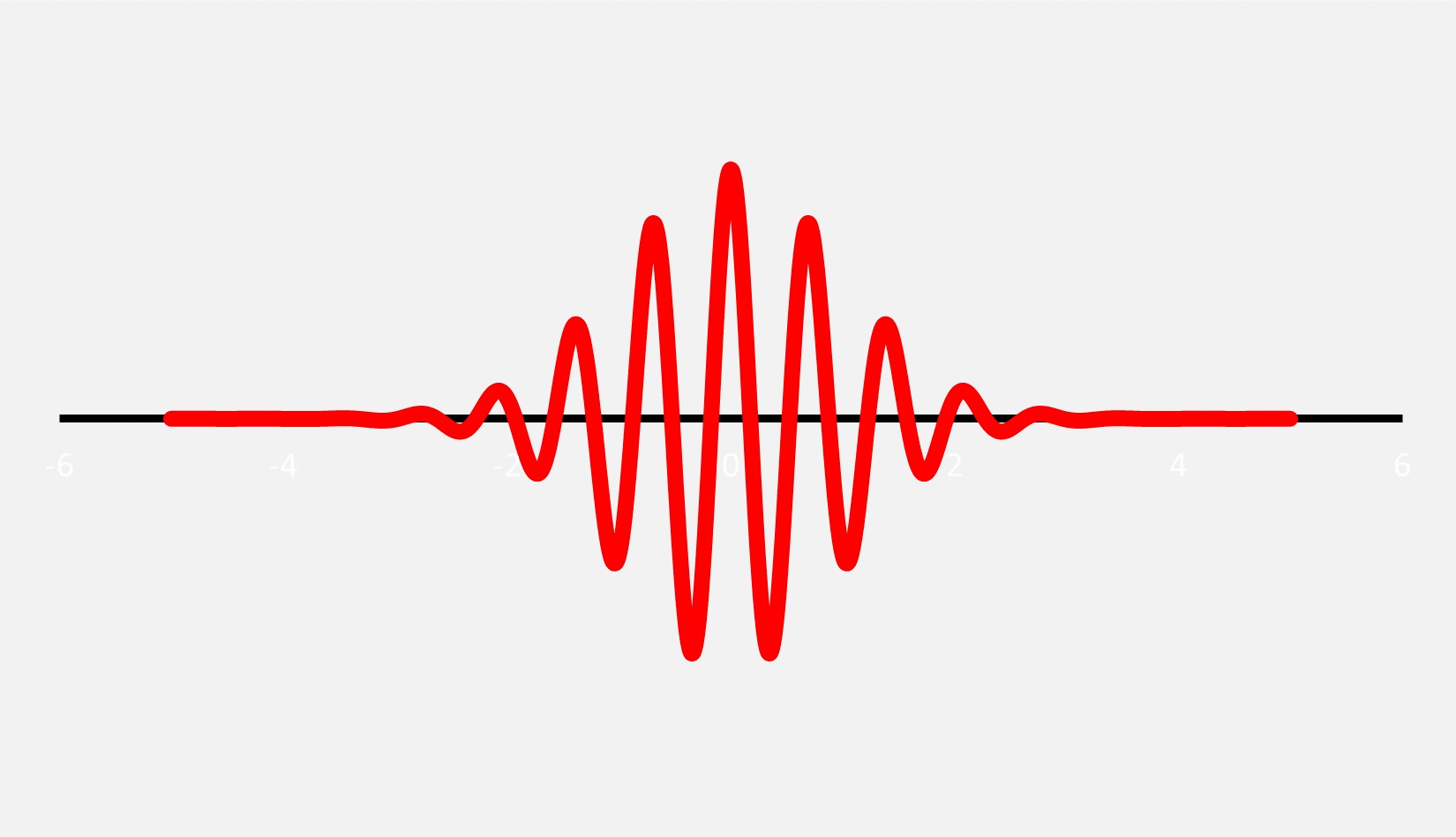
It is completely reasonable to think of subatomic particles like electrons and photons as wave packets, but given that waves are vibrations, one quickly asks, “What exactly is it that is vibrating?” or, equally confounding, “What is the meaning of the wave packet?” This is where things get a bit confusing.
In traditional quantum mechanics, this wave packet is called a wave function, and it is simply a method to calculate probabilities. If you square the wave function, the result is a function that tells you the likely locations where the particle will interact with other particles. This wave packet is merely a mathematical construct and nothing else.
Quantum field theory
However, the situation becomes somewhat more physical when more modern ideas of quantum mechanics are used. The name for the modern theory describing particles is “quantum field theory.” Modern quantum field theory postulates that space is full of a series of fields. There is a field for each kind of known subatomic particle. For example, there is an electron field, a photon field, and so on. There are even quark fields.
According to this theory, an electron is nothing more than a wave packet in the electron field. The meaning of the wave packet is the same as in traditional quantum mechanics — that is, if you square the wave function (representing the wave packet), the outcome is the probability of detecting an electron at that location.
The really neat thing about this understanding of particles is it gives us a very different mental picture of how particles are emitted and absorbed at the quantum level. For example, it is common for one subatomic particle to emit another, say, an electron emitting a photon. If subatomic particles are wave packets (localized vibrations of specific fields), then when an electron emits a photon, vibrations in the electron field are transferred to the photon field.
In a way, it’s like putting two identical tuning forks near one another and hitting one of them. The vibrations from that fork will transfer to the other, and soon both will be vibrating. In the quantum world, some of the vibrations of the electron field will transfer to the photon field, effectively creating a photon.
Good vibrations
There is no question that modern physics theories can be difficult to envision. However, once you have embraced the idea that particles are little more than localized vibrations in several interacting fields, you have a reasonably accurate vision of how the quantum world works.



